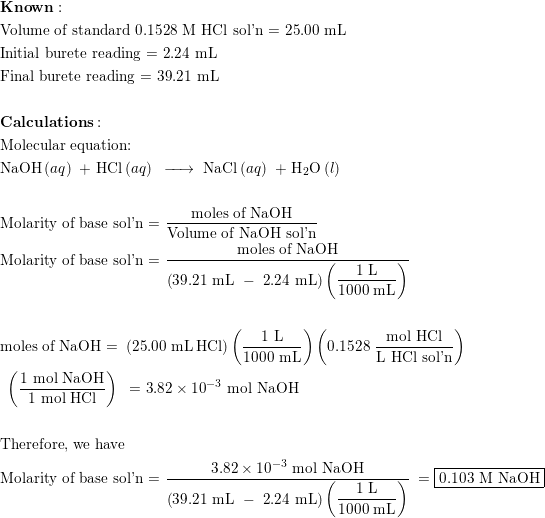
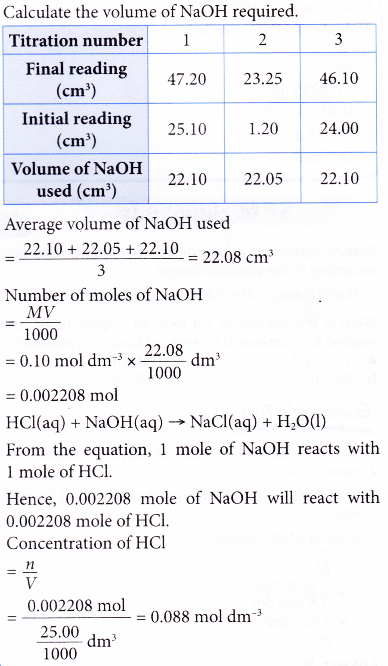
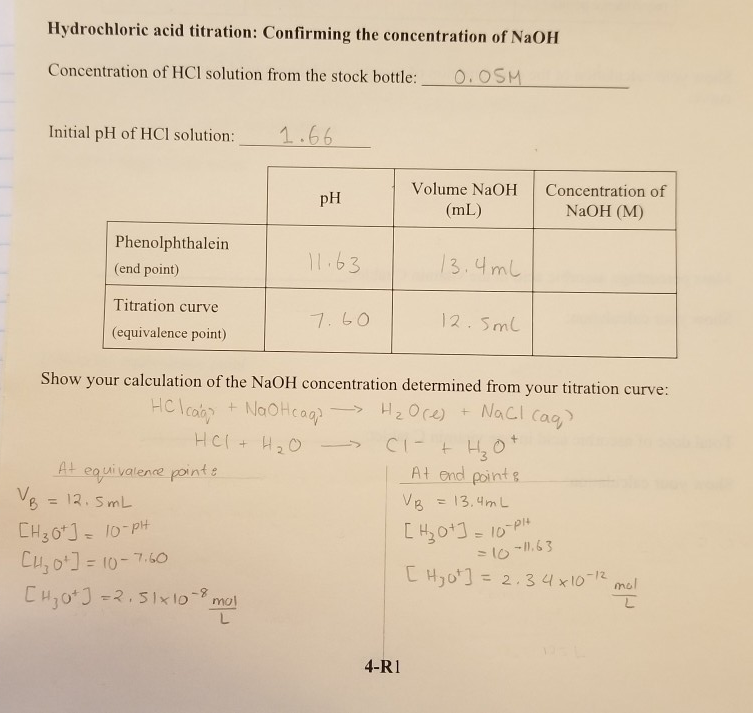
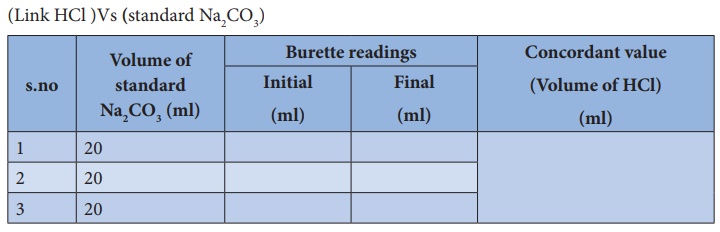
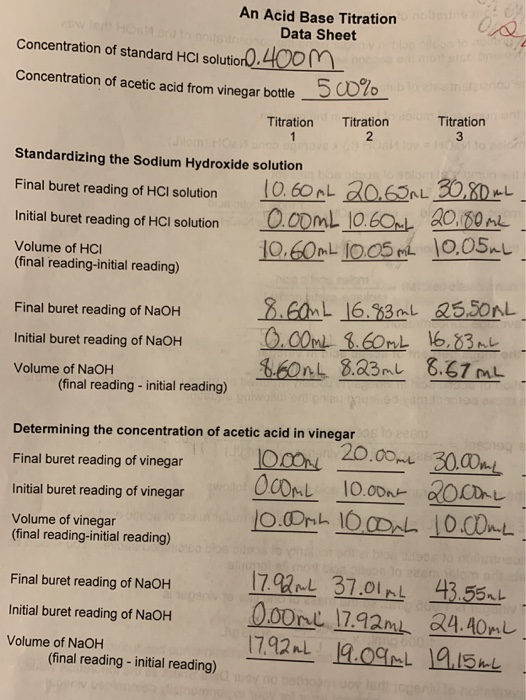
Calculate the concentration of HCl acid if 50 ml of HCl is required to neutralize 25 ml of 1 M NaOH in acid base titration.

SOLVED: 0.200 M sodium hydroxide (NaOH) being added to 30 mL of hydrochloric acid (HCl) of unknown concentration. Your goal is to measure the volume of sodium hydroxide needed to neutralize the
TITRATION Hydrochloric acid 0.1 mol/dm 3 Sodium hydroxide solution – concentration ? To determine the concentration of a solution of sodium hydroxide by. - ppt download
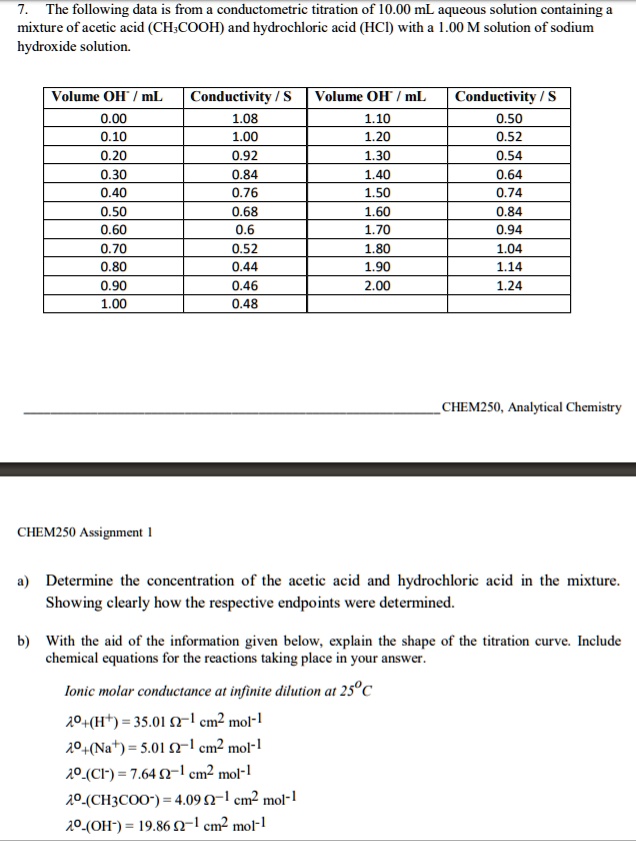
SOLVED: The following data is from conductometric titration of 10.00 mL aqueous solution containing mixture of acetic acid (CHCOOH) and hydrochloric acid (HCI) with 1.00 M solution of sodium hydroxide solution Volume
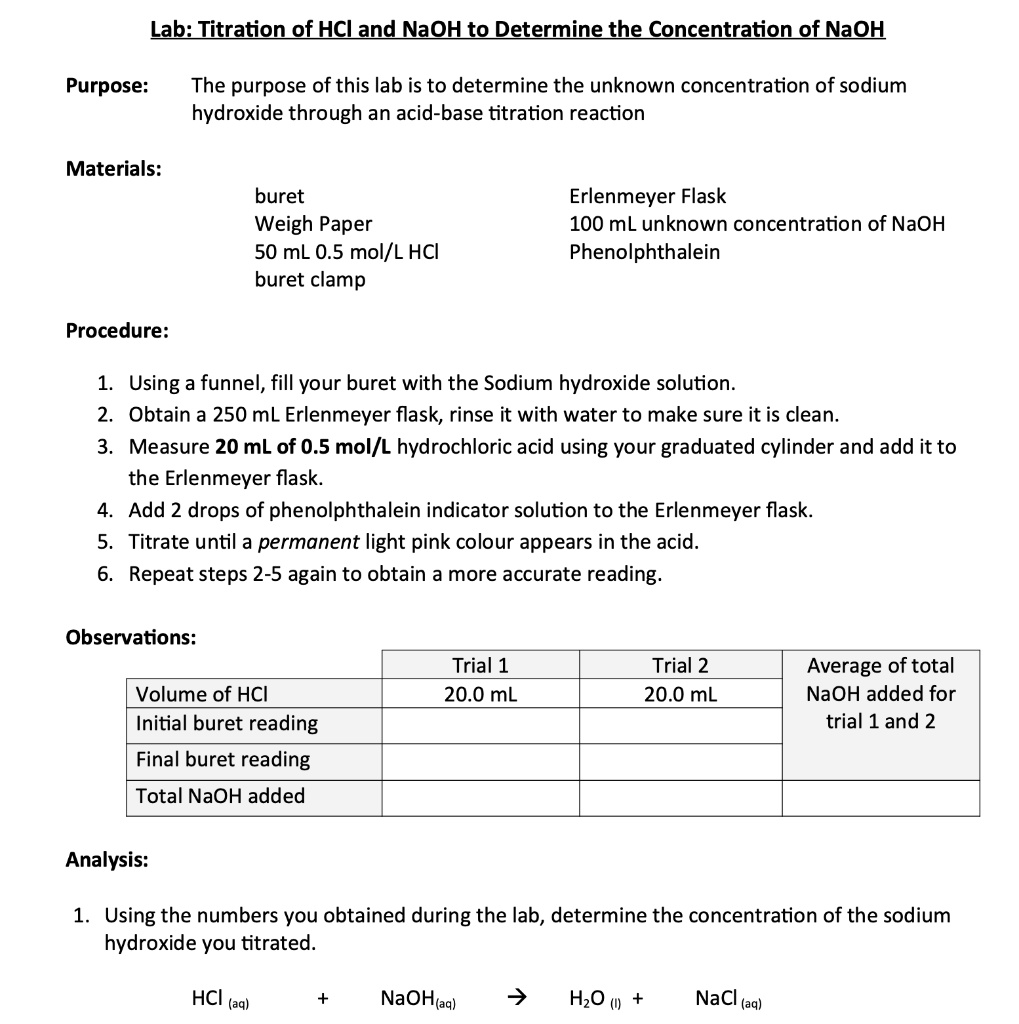
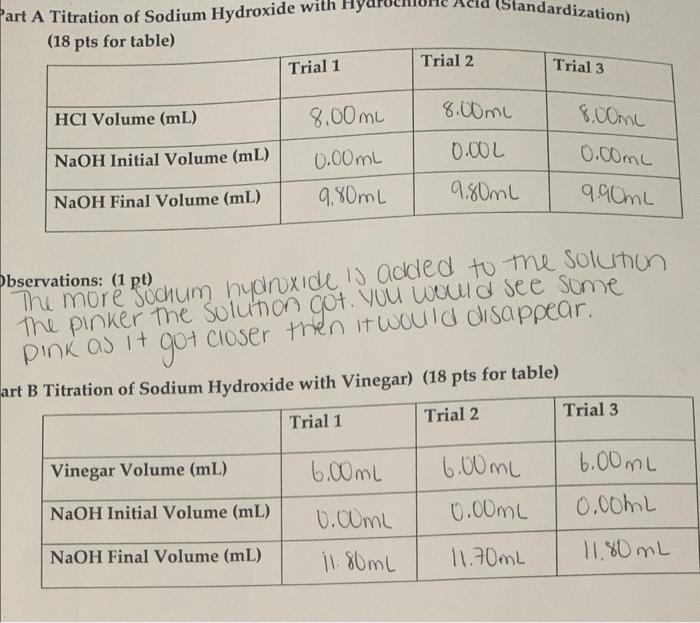
SOLVED: Lab: Titration of HCLand NaOHto Determine the Concentration of NaOH Purpose: The purpose of this lab is to determine the unknown concentration of sodium hydroxide through an acid-base titration reaction Materials:

SOLVED: Molarities of Solution:: Solution Molarity NAOH LlolsM HCI MldleH HC,HO: L10XM Titration of Hydrochloric Acid: mL base needed to reach the equivalence point: 44Im Show how you detenined this volume from

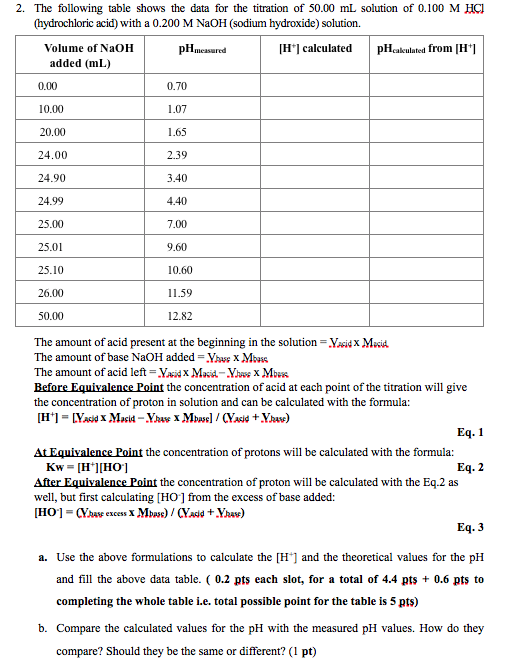
Here is an example of a titration curve, produced when a strong base is added to a strong acid. This curve shows how pH varies as 0.100 M NaOH is added to 50.0 mL of 0.100 M HCl.