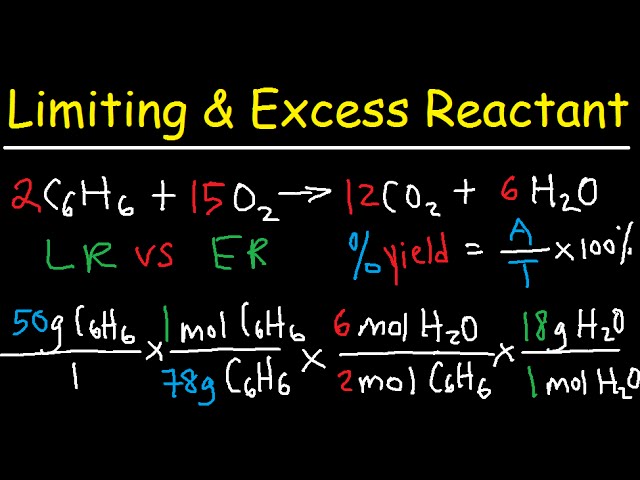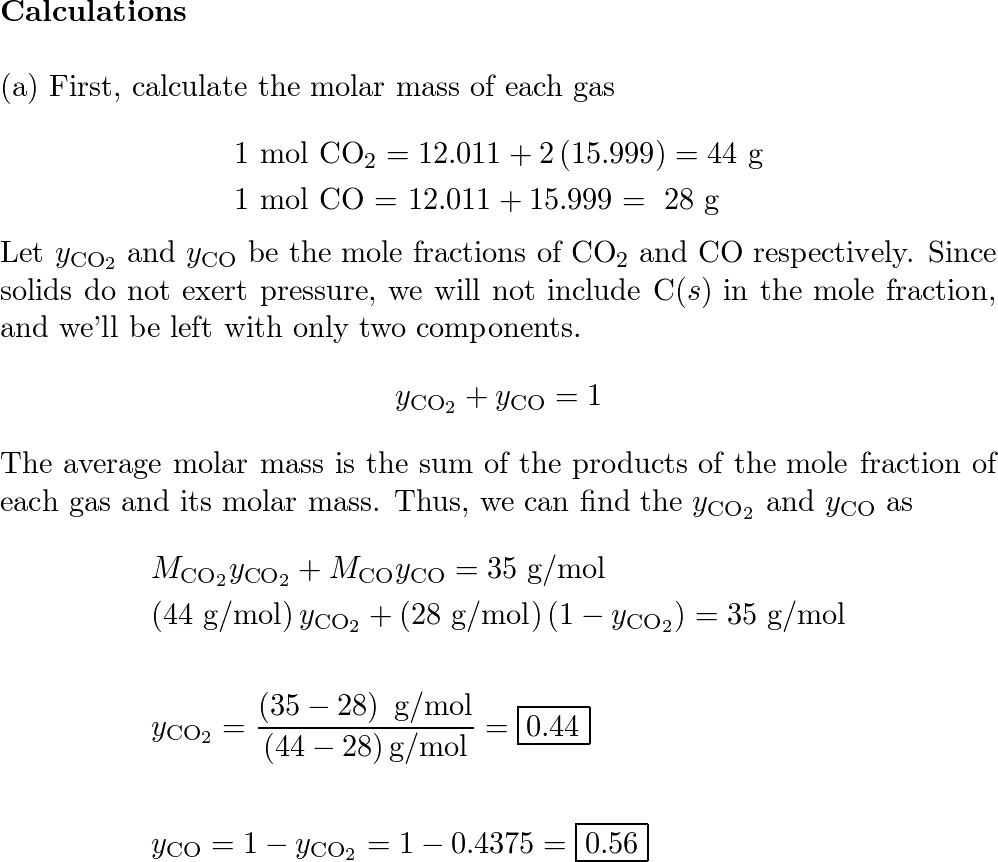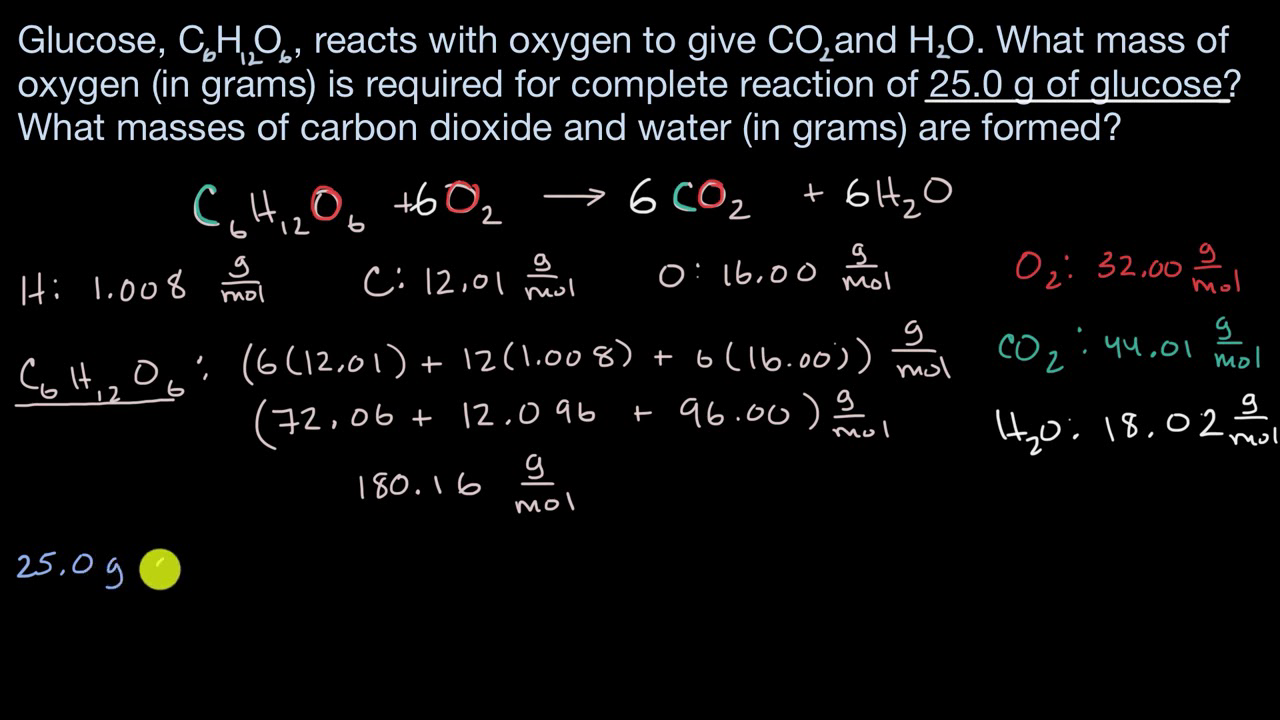Fluorine reacts with uranium to form UF6 . U(s) + 3F2(g) → UF6(g) How many fluorine molecules are required to produce 2 mg of UF6 from an excess of uranium? The molar

When 7.45 g of metal chloride dissolved in excess of water, the amount of heat absorbed is X kJ. Calculate the enthalpy of solution if molar mass of chloride is 74.5.

PDF) Calculation of excess enthalpy of binary mixtures with using of excess volume experimental data

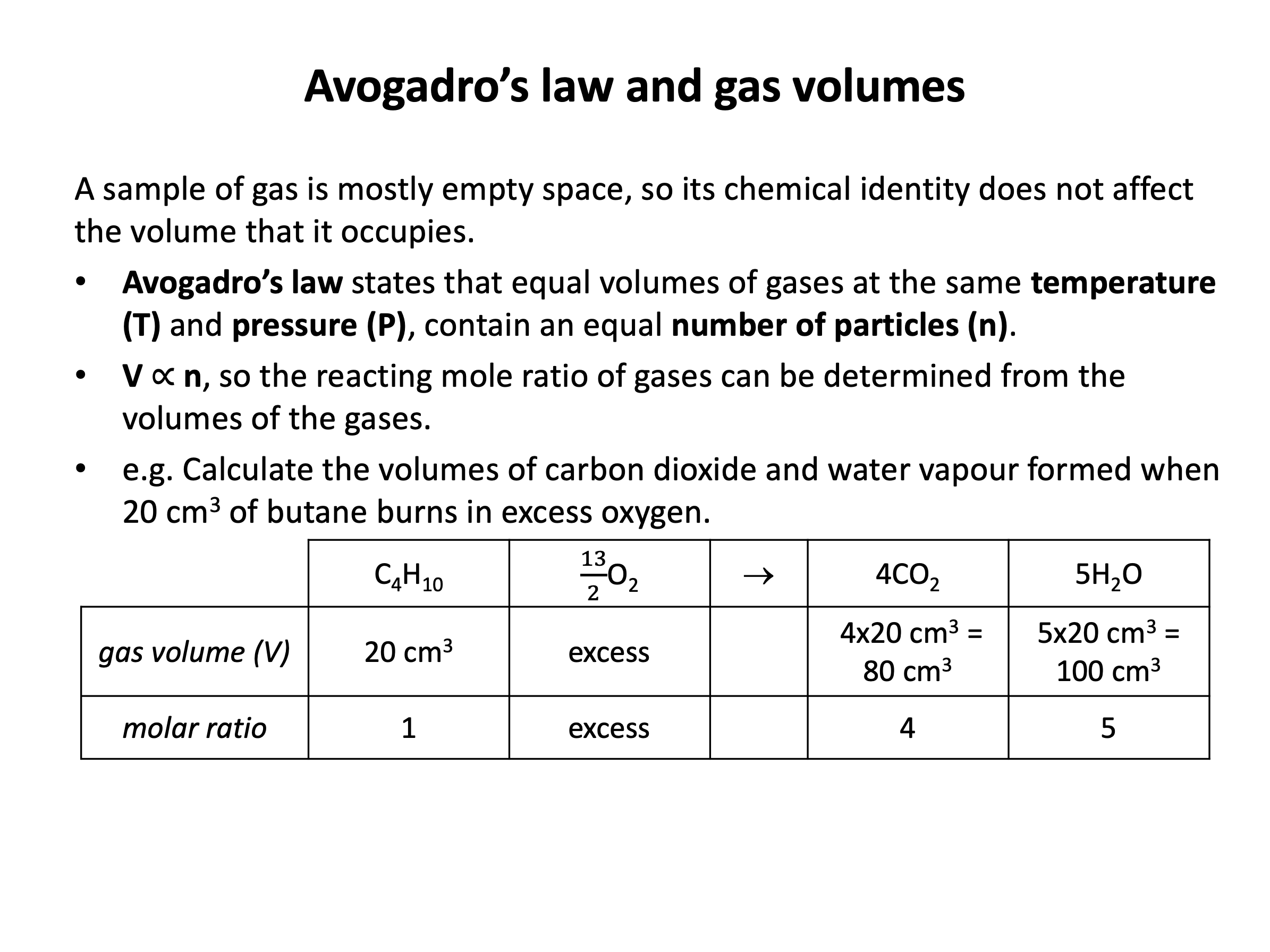
molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

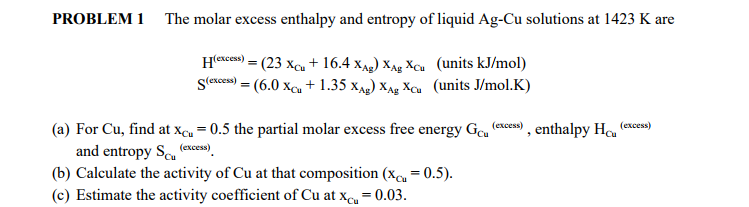
SOLVED: The molar excess Gibbs free energy of formation of solid solutions in the 'system Au-Ni can be represented by G*s '=XiXAu(QAIAOX Au + 38280X Ni 14230XAuX Ni 2660 Calculate the activities

Calculating the Heat of Reaction from Molar Reaction Enthalpy and the Mass of a Reactant | Chemistry | Study.com
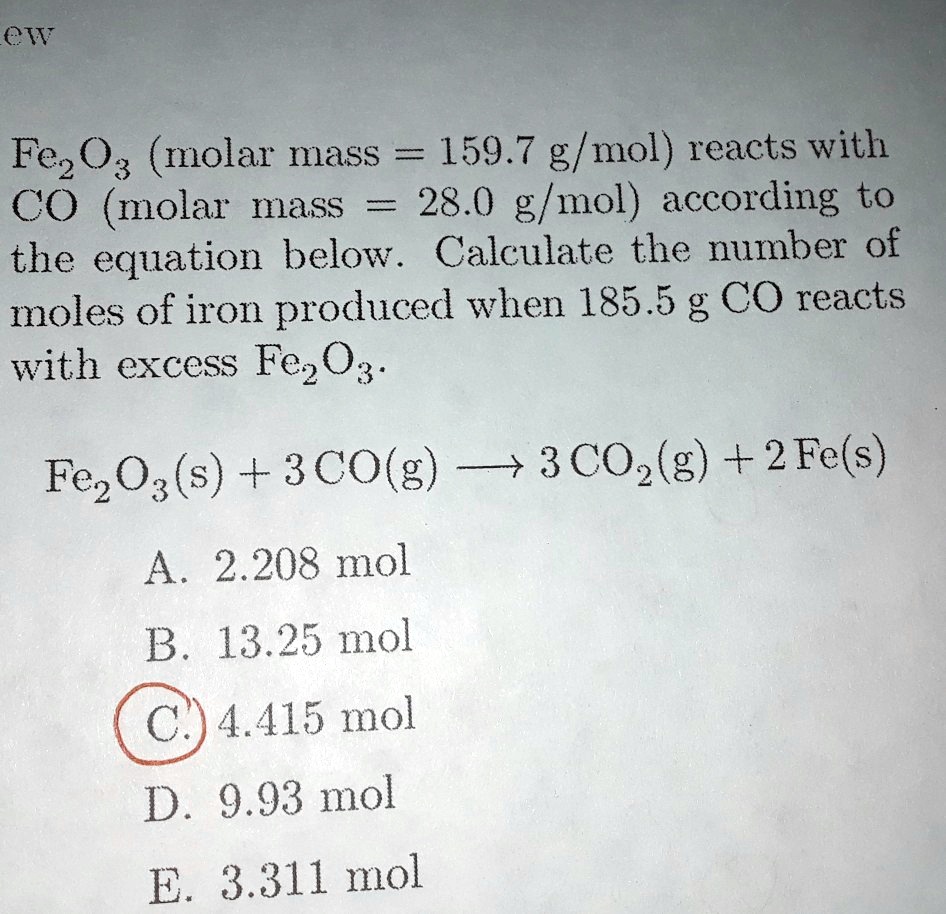
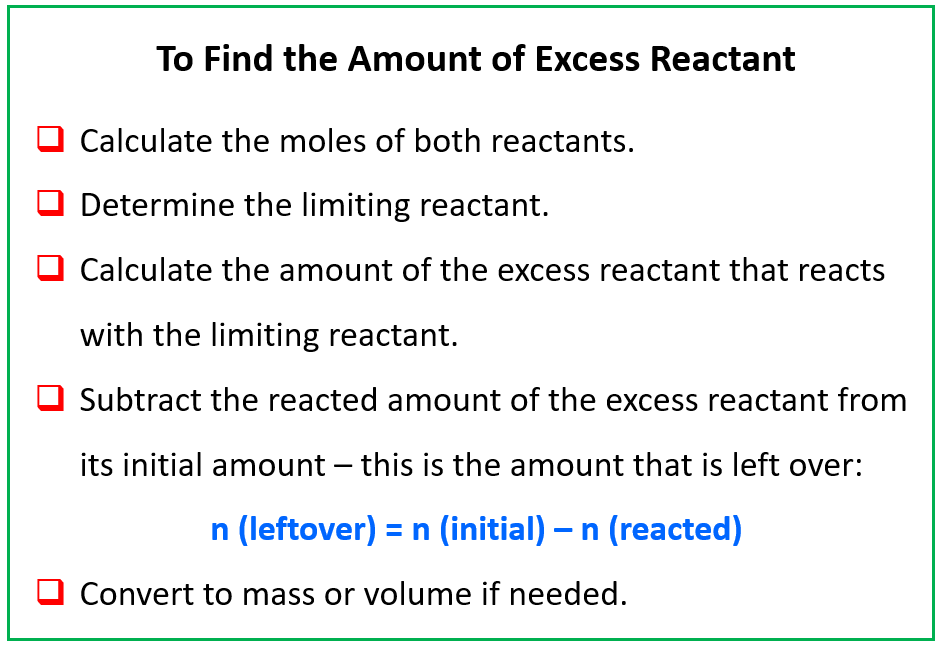
SOLVED: ew Fez O3 (molar mass = 159.7 g/mol) reacts with CO molar mass 28.0 g/mol) according to the equation below. Calculate the number of moles of iron produced when 185.5 g