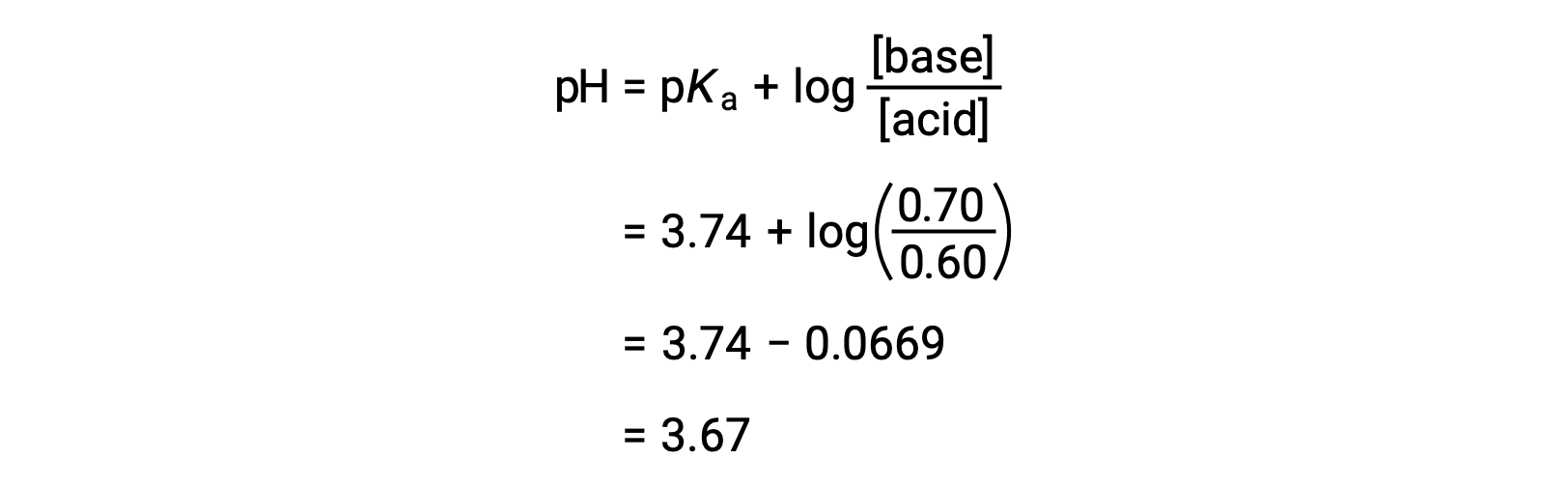Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
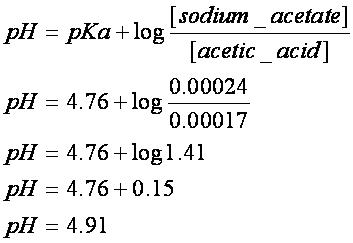
pH calculations and more in fundamentals of pharmaceutics. : Calculate pH of 100 ml buffer solution containing 0.1 g acetic acid and 0.2 g sodium actetate.

OneClass: Calculating pH Change For a Buffer Calculate the pH after 1.0 g of Mg(OH)_2 is added to 155...
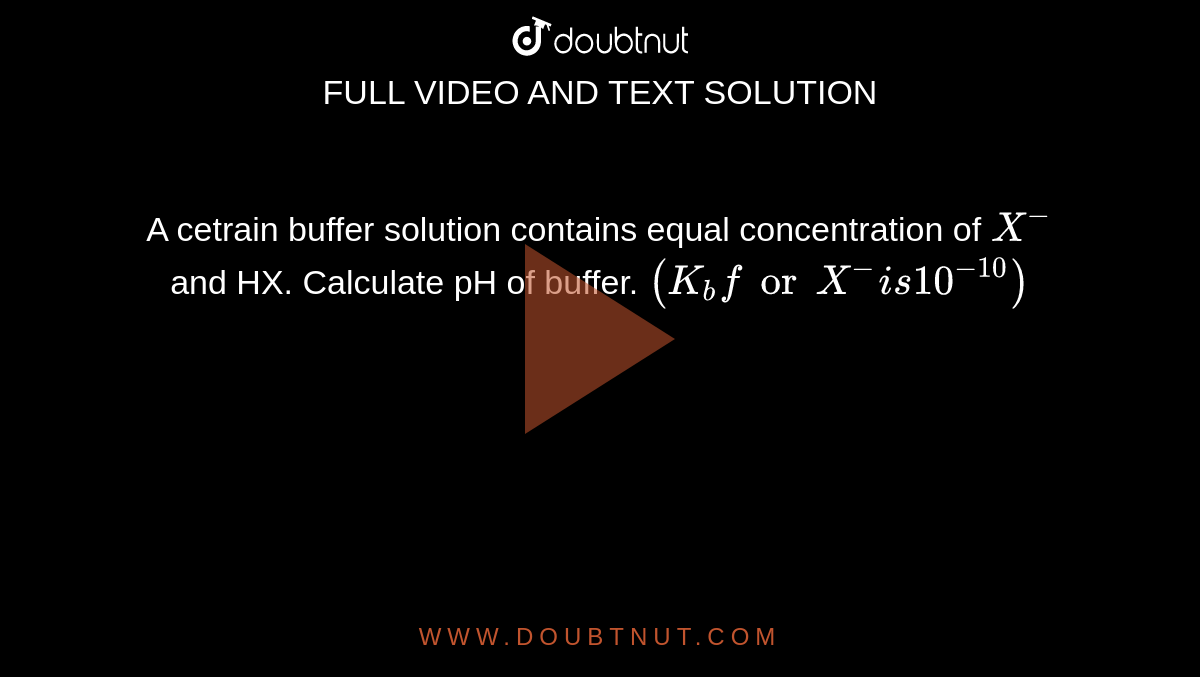
A cetrain buffer solution contains equal concentration of X^(-) and HX. Calculate pH of buffer. (K(b)for X^(-)is 10^(-10))

Calculate the PH of a buffer solution prepared by dissolving 30g of Na2CO3 in 500 ml of an aqueous solution containing 150 ml of 1m HCL . ka for HCO^-3 = 5.63 x 10 - 11

Calculate the pH of buffersolution containing 0.05 mol NaF per litreand 0.015 mol HF per litre. [K= - Brainly.in

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com

pH calculations and more in fundamentals of pharmaceutics. : Calculate pH and buffer capacity of a carbonic acid/ bicarbonate buffer solution.