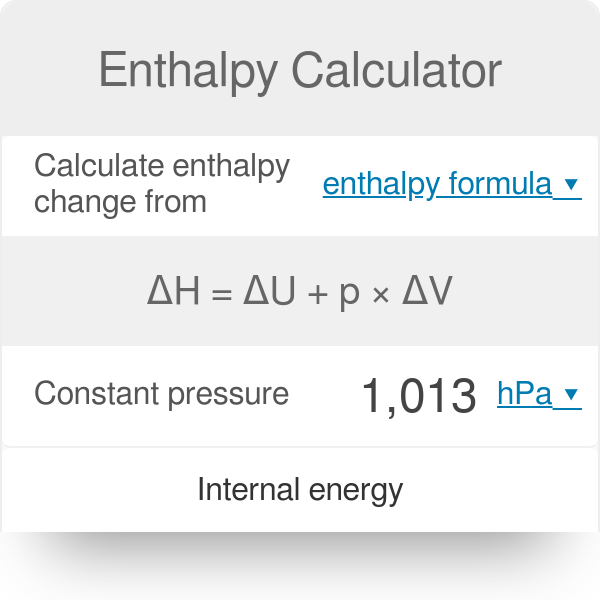
Question Video: Determining a Standard Enthalpy Change Given the Standard Enthlapy of Fusion | Nagwa
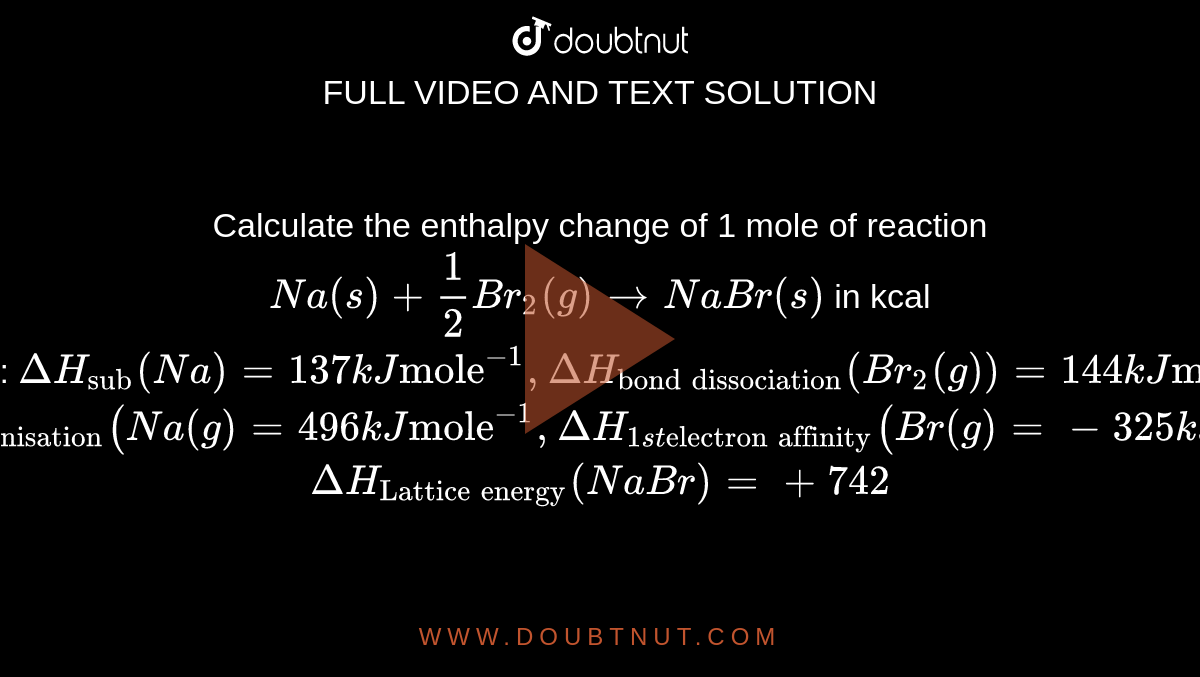
Calculate the enthalpy change of 1 mole of reaction Na(s)+(1)/(2)Br(2)(g)rarrNaBr(s) in kcal Given : Delta H("sub")(Na)=137 kJ "mole"^(-1) , DeltaH("bond dissociation")(Br(2)(g))=144 kJ "mole"^(-1) Delta H("1 st ionisation")(Na(g)=496 kJ "mole"^(-1 ...


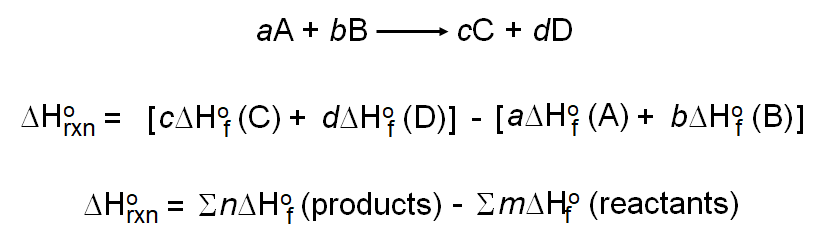

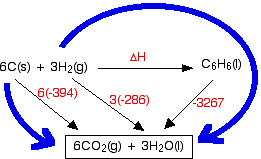

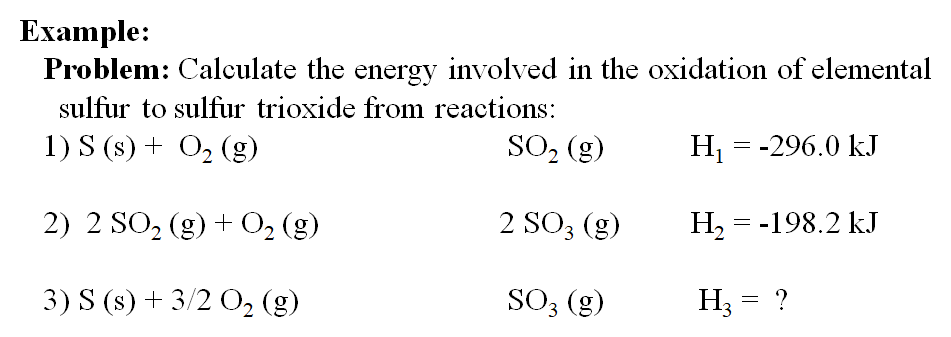



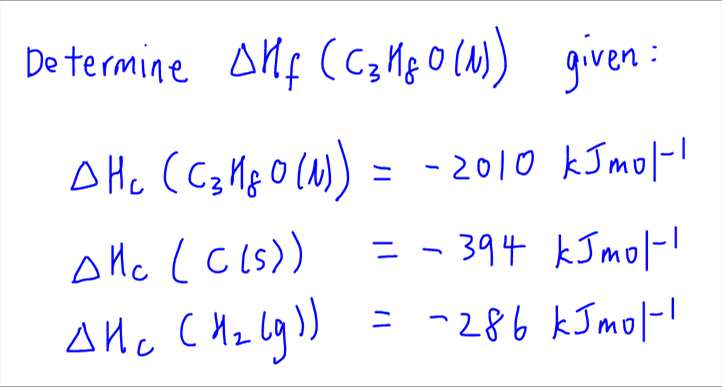

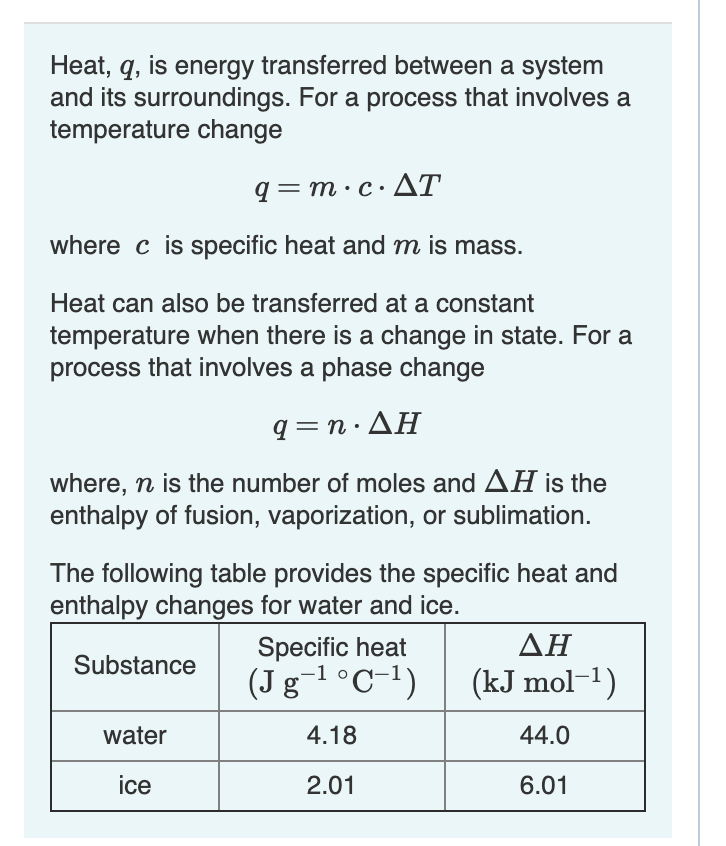

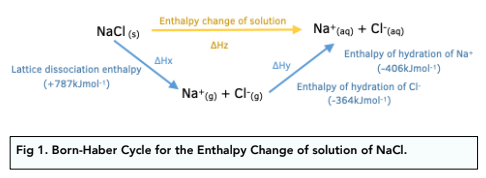


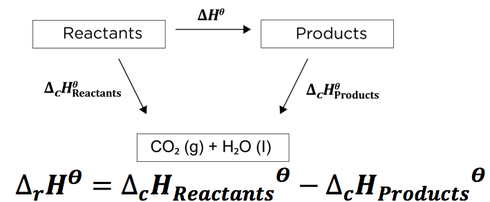

![Example] How to Calculate Enthalpy Change of a Reaction. - YouTube Example] How to Calculate Enthalpy Change of a Reaction. - YouTube](https://i.ytimg.com/vi/nmNQUGt6NiM/maxresdefault.jpg)