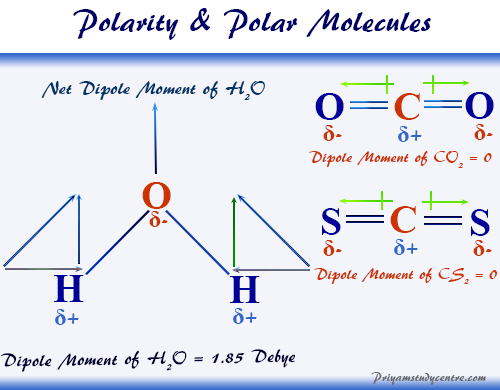
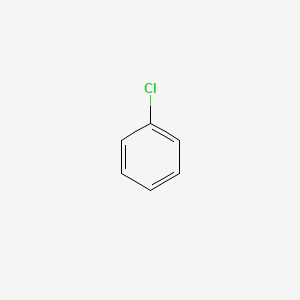
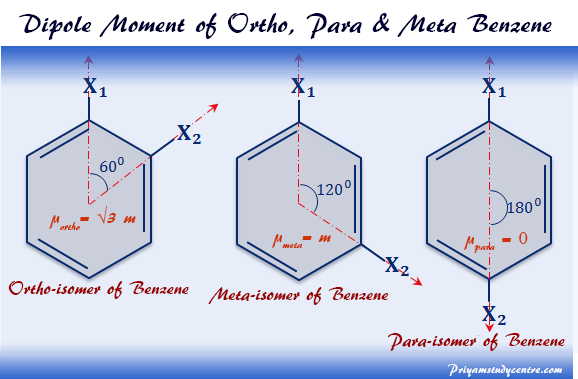
Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru

Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru

Table 1 from A dispersive liquid-liquid microextraction using a switchable polarity dispersive solvent. Automated HPLC-FLD determination of ofloxacin in chicken meat. | Semantic Scholar

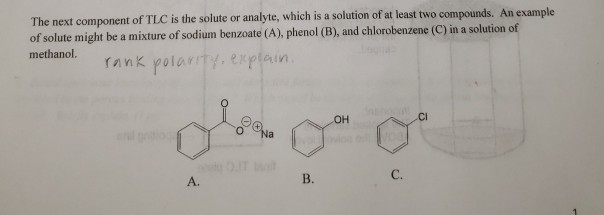
The elution sequence of a mixture of compounds containing chlorobenzene, anthracene and p-cresol developed on an alumina column using a solvent system of progressively increasing polarity is (a) anthracene to chlorobenzene to