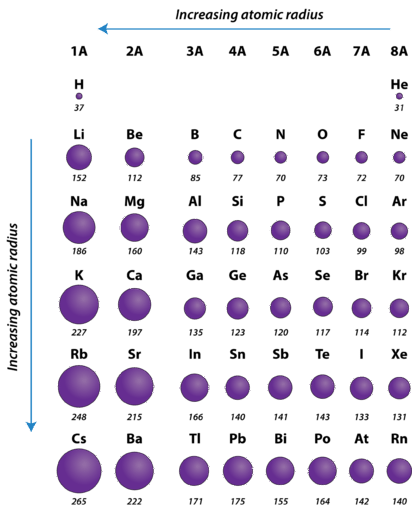
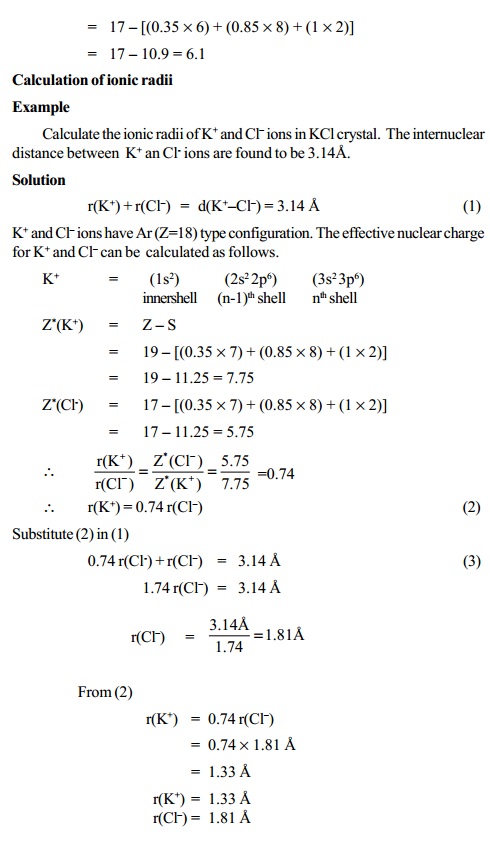
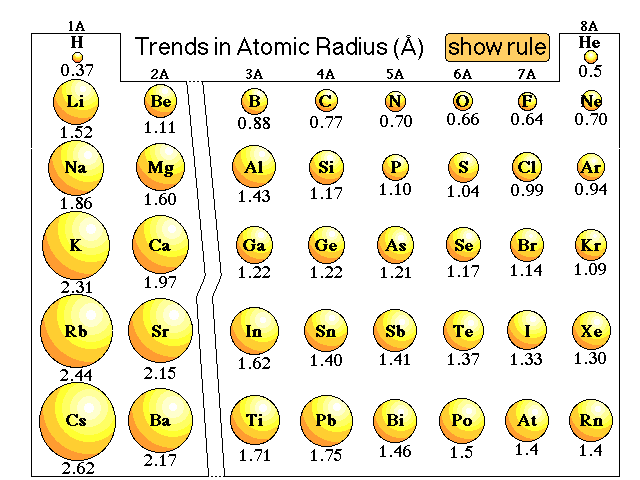
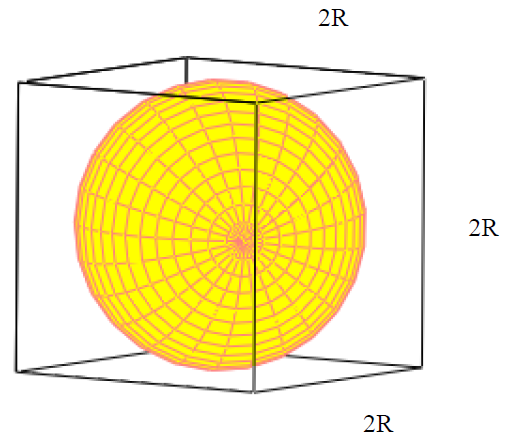
Lithium metal crystallizes in a body - centered cubic crystal. If the length of the side of the unit cell of lithium is 351 pm, the atomic radius of lithium will be:

Ionic Radius Trends, Basic Introduction, Periodic Table, Sizes of Isoelectric Ions, Chemistry - YouTube

Niobium crystallises in body - centred cubic structure. If density is 8.55 g cm^-3 , calculate atomic radius of niobium using its atomic mass 93 U .

Calculate the radius of He atoms if its van der Waal's constant 'b' is 24mL `"mol"^(-1)`. (Note: mL= - YouTube
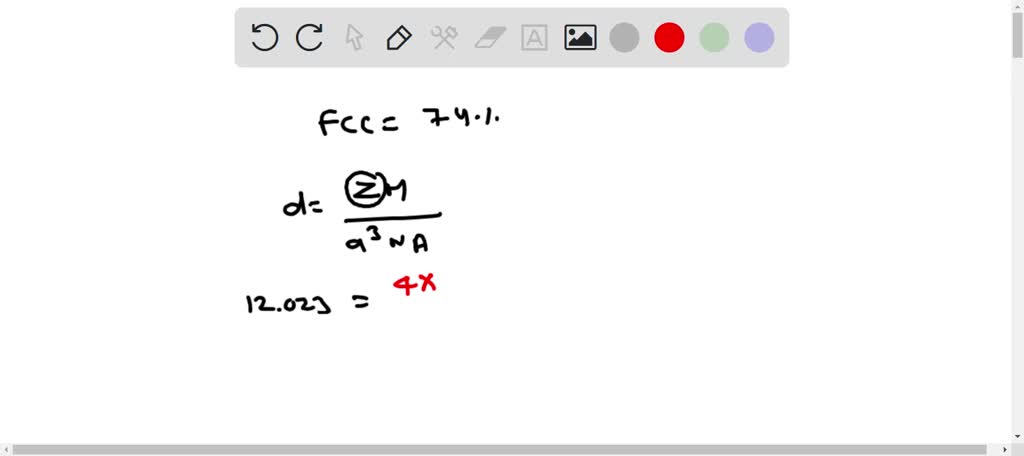
SOLVED: Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3 . Calculate the atomic radius of palladium and its packing efficiency.