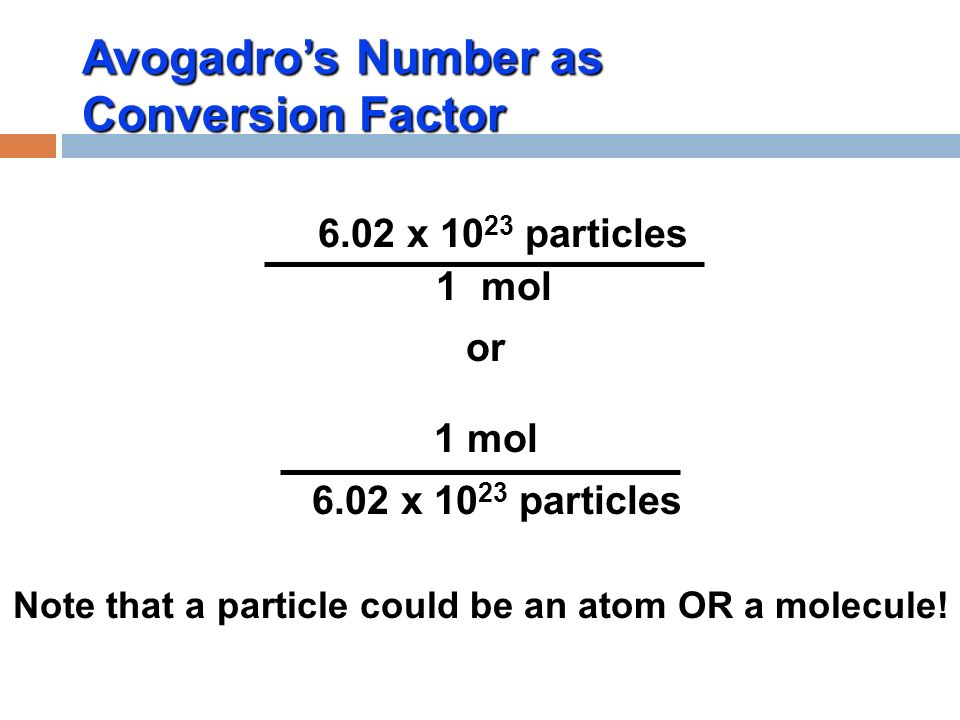
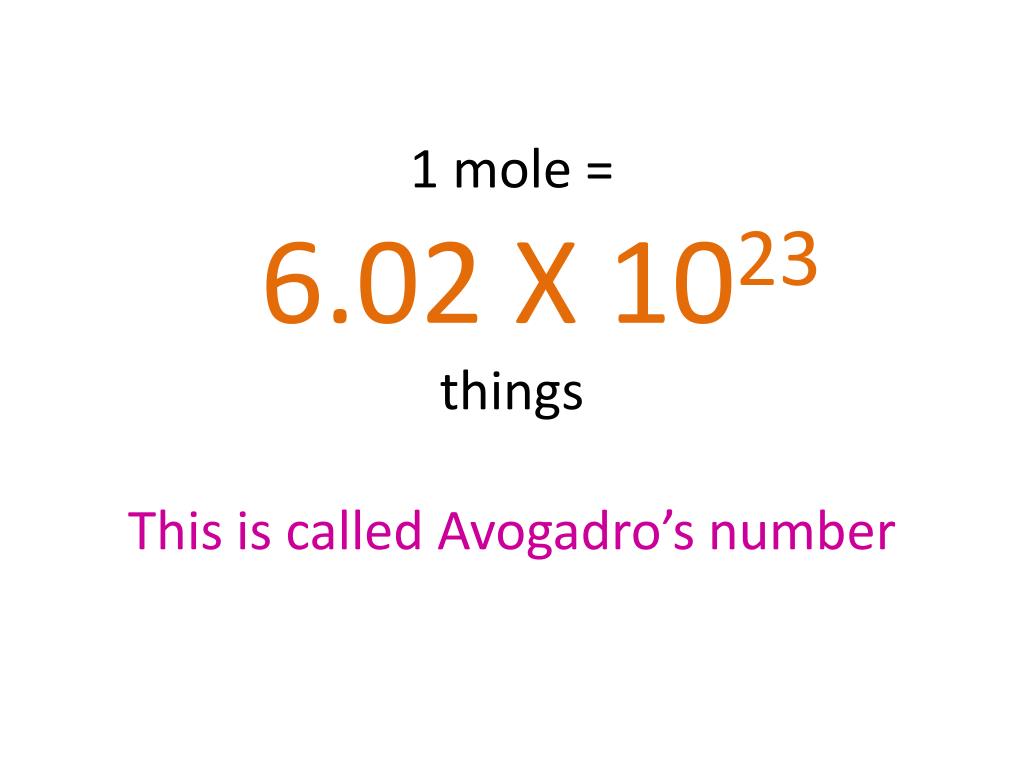The Mole Concept. What is a mole? IIn chemistry, a mole is a counting unit. Abbreviated mol. 11 mol = 6.022x10 23 representative particles. Avogadro's. - ppt download
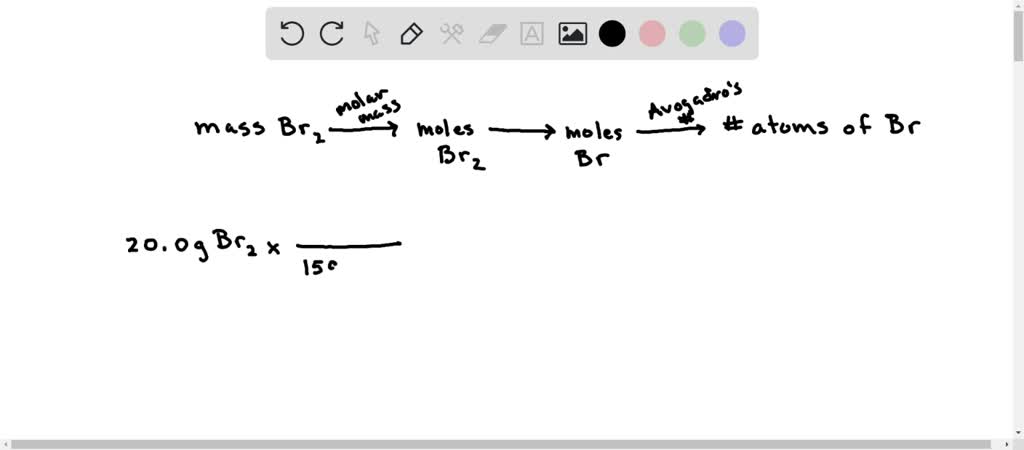
SOLVED: Calculate the number of Br atoms in 20.0g of liquid bromine (Br2). NOTE: Avogadro's number is 6.02 x 1023 A 7.53*10^23 B. 1.51*10^23 C. 3.01*10 ^23 D. 3.77*10^23 E. Nor correct response

Lithium has a BCC structure. Its density is 530 kg m^-3 and its atomic mass is 6.94 g mol^-1 . Calculate the edge length of a unit cell of Lithium metal: ( NA = 6.02 × 10^23 mol^-1 )

12 g C - 12 contains 6.022 × 10^23 atoms of carbon.(a) 6.022 × 10^23 is known as .............(b) Calculate the number of carbon atoms present in 48 g C - 12.(c)